| The Problem: |
| Activity 1_2 makes evident that different temperatures are measured in different places of our house model, where an heater is turned on. Now we will observe what happens in a basin where different sides are at different temperatures. |
| Learning aims: |
|
• To be aware that temperature gradients in fluids produce convective currents. • To identify mechanisms of functioning” on the base of density differences among fluid volumes at different temperatures and buoyancy properties. |
| Materials: |
|
• Two bowls filled of hot water and ice, respectively. • A small fish tank filled with water at room temperature. • Two small amount of red and blue dyes. |
| Suggestions for use: |
   |
| Figure 3_1a) Figure 3_1b) Figure 3_1c) |
| The fish tank filled with water is placed over the two bowls (see Fig. 3_1a) and two drops of red and blue dyes are gently posed on the tank surfaces (see Fig. 3_1b). At the following web site (http://www.youtube.com/watch?v=7xWWowXtuvA&feature=related) you can see the video of the experiment.The teacher can perform the demonstration and put questions recalling every days phenomena stimulating students to identify variations of density in equal volumes of the same fluid at different temperatures and the consequent upward movement of hot fluid. |
| Possible questions: |
|
1. What happens if we put a drop of oil at the bottom of a basin containing water? Why? 2. Analyse the heating of a pot of water on a stove and describe what happens.
|
|
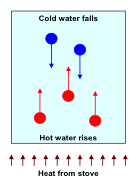
NOTE: A well known example of heat transfer by convection is provided by the heating of a pot of water on a stove. Turning on the stove thermal energy is transferred first by conduction from the stove to the bottom of the pot and from this latter to the water. After a given time some bubbles of hot water on the bottom of the pot appear. These bubbles, that actually are local regions of hot water less dense than the cold one, rise to the surface and by the mechanism of convection transfer heat from the hot water, at the bottom, to the cold water, at the top. At the same time, the cold water at the top, denser then the hot one, falls to the bottom and is heated to this latter. The following figure shows the convection currents.
|
|
3. Why does this balloon move upward?
The figure shows a popular toy The hot air balloon: when the candle is lighted the balloon begins to move upward. Can you explain why? |
|
NOTE By analysing students’ answers to posed questions, the following kind of mechanism can be hypothesized. Suppose we consider heating up a local region of air. As this air heats, the molecules spread out, causing this region to become less dense than the surrounding, unheated air. As a consequence, being less dense than the surrounding cooler air, the hot air will rise due to buoyant forces and this movement of hot air into a cooler region will transfer energy by heating the cooler regions. |
|
Further questions can be posed through the analysis of the following phenomenon:
Phenomena of breezes over land masses near to large basins of water supply a relevant example of convection currents. Water has a larger heat capacity than land. As a consequence it holds heat better than land and takes longer to change its temperature, either upward or downward. Thus, in the morning, due to the sun heating, the air above the water is cooler than that over the land. This creates a low pressure area over the land, with respect to the high pressure area over the water. Due to this pressure difference air is pushed from the water to the land as a blowing breeze. On the other hand, during the night water cools off more slowly than the land, and the air above the water is slightly warmer than over the land. This produces a low pressure area over the water with respect to the high pressure area over the land, and this time air is pushed from the land to the water. |