Chitosan according to its structure and properties has many possibilities of application. Bader and Birkholz (1997) published the following list:
- The clarification and cleaning of protein-containing waste water of fruit, meat, fish and milk industry as well as of breweries were the biggest and for a long time the only use of these polysaccharides. Chitosan causes the coagulation of the proteins found in waste water. As a naturally occurring polymer the chitosan is degradable and non-toxic, so it should be preferable to synthetic polymers.
- Analogously, fruit and vegetable juice are clarified with the aid of chitosan.
- Chitosan and chitin are chelating agents showing complexing ability for many metal ions. The cations coordinate to free electron pairs of nitrogen and oxygen atoms. The affinity of chitosan to metal ions obeys the following order: Cr3+ < Co2+ < Pb2+ < Mn2+ « Cd2+ < Ag+ < Ni2+ < Fe3+ < Cu2+ < Hg2+. With the aid of appropriate acids (e.g. diluted sulphuric acid) chitosan is regenerated and reused. One field of application is the purification of heavy-metal contaminated waste water.
- Membranes made of chitosan are suitable for water softening, because they are impermeable to calcium ions.
- Paper impregnated with a solution containing 3 % chitosan shows a significant higher tear resistance, abrasion resistance and moisture resistance compared to untreated paper.
- Due to the antibacterial character of chitosan, it is possible to use packaging films of chitosan for preservation.
- In technology chitin and chitosan are used for the production of membranes, fibres and films. At present composites, e.g. materials partly made of cellulose, are studied. Chitosan has an outstanding film-forming property caused by intra- and intermolecular hydrogen bonding.
Chemical modification of chitin and chitosan leads to further applications, just to mention a few:
- Since chitin, chitosan and different derivatives of these compounds are degradable by endogenous enzymes and have no allergic effects, they are used in different medical and pharmaceutical fields. Examples are suture materials, wound dressings as well as synthetic skin.
- Recently, derivatives of chitosan are used in hair-care products, thanks to their setting, conditioning and caring properties. In creams and ointments chitosan derivatives are used because of their water-binding ability and adhesiveness.
- Chitosan salts are formed by reaction of chitosan with a multitude of inorganic and organic acids. For example, if chitosan is heated to boiling in the presence of hydrochloric acid, water soluble chitosan hydrochloride precipitates on cooling, which is one of the starting materials in the cosmetics.
- Coating with a film made of N,0-carboxymethyl chitosan improves the storage stability of seeds and fruits. The low oxygen permeability and the antibacterial effect of these films guarantee preservation for a long time.
- Under basic conditions chitosan reacts with alkyl halides to N,0-alkylchitosan. So the reaction with chloroacetic acid yields water soluble N,0-carboxymethyl chitosan. This can be used for films.
There are several more possibilities of application. During this course we will focus on chitosan as slimming agent. Because of its property to bind 8 times its weight of fat it is advertised as slimming agent or "Fat Magnet". In an acidic milieu the amino groups will be protonized and thus charged positively. These poly-kations are able to bind the negatively charged fatty acid anions, what is irreversible and the fat cannot be metabolized and so the captured fat leaves the body undigested and does not enter the organism. When less fat is available for the organism, the body draws the necessary fat from its fat reserves, which automatically leads to a loss of weight.
But:
Pharmaceutical studies show no positive effect of chitosan for weight loss (Google: chitosan Pharmaceutical studies): E.g.: "The new study, published in the September issue of the International Journal of Obesity (28, 1149-1156), is one of the largest to date. The researchers assigned 250 adults, with an average body mass index of 35.5, to receive either 3g of chitosan daily or a placebo for 24 weeks. All participants received standardised dietary and lifestyle advice for weight loss. The researchers from the Clinical Trials Research Unit in the University of Auckland report that the chitosan group lost more body weight than the placebo group "but the effects were small". The chitosan group lost an average of 0.4kg compared to a 0.2 kg gain in the placebo group." (24.08.2004: http://www.nutraingredients-usa.com/news/ng.asp?id=54318-chitosan-fails-to)
Chitosan according to its structure and properties has many possibilities of application. Bader and Birkholz (1997) published the following list:
- The clarification and cleaning of protein-containing waste water of fruit, meat, fish and milk industry as well as of breweries were the biggest and for a long time the only use of these polysaccharides. Chitosan causes the coagulation of the proteins found in waste water. As a naturally occurring polymer the chitosan is degradable and non-toxic, so it should be preferable to synthetic polymers.
- Analogously, fruit and vegetable juice are clarified with the aid of chitosan.
- Chitosan and chitin are chelating agents showing complexing ability for many metal ions. The cations coordinate to free electron pairs of nitrogen and oxygen atoms. The affinity of chitosan to metal ions obeys the following order: Cr3+ < Co2+ < Pb2+ < Mn2+ « Cd2+ < Ag+ < Ni2+ < Fe3+ < Cu2+ < Hg2+. With the aid of appropriate acids (e.g. diluted sulphuric acid) chitosan is regenerated and reused. One field of application is the purification of heavy-metal contaminated waste water.
- Membranes made of chitosan are suitable for water softening, because they are impermeable to calcium ions.
- Paper impregnated with a solution containing 3 % chitosan shows a significant higher tear resistance, abrasion resistance and moisture resistance compared to untreated paper.
- Due to the antibacterial character of chitosan, it is possible to use packaging films of chitosan for preservation.
- In technology chitin and chitosan are used for the production of membranes, fibres and films. At present composites, e.g. materials partly made of cellulose, are studied. Chitosan has an outstanding film-forming property caused by intra- and intermolecular hydrogen bonding.
Chemical modification of chitin and chitosan leads to further applications, just to mention a few:
- Since chitin, chitosan and different derivatives of these compounds are degradable by endogenous enzymes and have no allergic effects, they are used in different medical and pharmaceutical fields. Examples are suture materials, wound dressings as well as synthetic skin.
- Recently, derivatives of chitosan are used in hair-care products, thanks to their setting, conditioning and caring properties. In creams and ointments chitosan derivatives are used because of their water-binding ability and adhesiveness.
- Chitosan salts are formed by reaction of chitosan with a multitude of inorganic and organic acids. For example, if chitosan is heated to boiling in the presence of hydrochloric acid, water soluble chitosan hydrochloride precipitates on cooling, which is one of the starting materials in the cosmetics.
- Coating with a film made of N,0-carboxymethyl chitosan improves the storage stability of seeds and fruits. The low oxygen permeability and the antibacterial effect of these films guarantee preservation for a long time.
- Under basic conditions chitosan reacts with alkyl halides to N,0-alkylchitosan. So the reaction with chloroacetic acid yields water soluble N,0-carboxymethyl chitosan. This can be used for films.
There are several more possibilities of application. During this course we will focus on chitosan as slimming agent. Because of its property to bind 8 times its weight of fat it is advertised as slimming agent or "Fat Magnet". In an acidic milieu the amino groups will be protonized and thus charged positively. These poly-kations are able to bind the negatively charged fatty acid anions, what is irreversible and the fat cannot be metabolized and so the captured fat leaves the body undigested and does not enter the organism. When less fat is available for the organism, the body draws the necessary fat from its fat reserves, which automatically leads to a loss of weight.
But:
Pharmaceutical studies show no positive effect of chitosan for weight loss (Google: chitosan Pharmaceutical studies): E.g.: "The new study, published in the September issue of the International Journal of Obesity (28, 1149-1156), is one of the largest to date. The researchers assigned 250 adults, with an average body mass index of 35.5, to receive either 3g of chitosan daily or a placebo for 24 weeks. All participants received standardised dietary and lifestyle advice for weight loss. The researchers from the Clinical Trials Research Unit in the University of Auckland report that the chitosan group lost more body weight than the placebo group "but the effects were small". The chitosan group lost an average of 0.4kg compared to a 0.2 kg gain in the placebo group." (24.08.2004: http://www.nutraingredients-usa.com/news/ng.asp?id=54318-chitosan-fails-to)
This module will focus mainly on the application of chitosan as slimming aid.
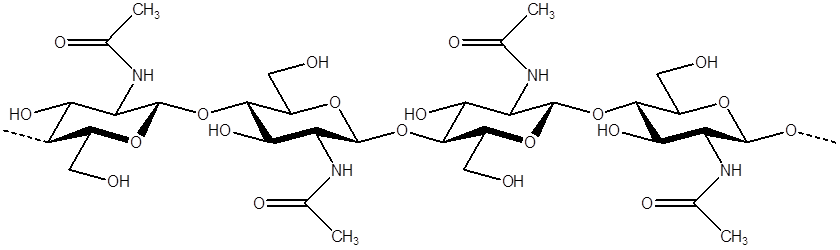
Chitin: Poly-β-1,4-N-acetyl-D-glucosamin
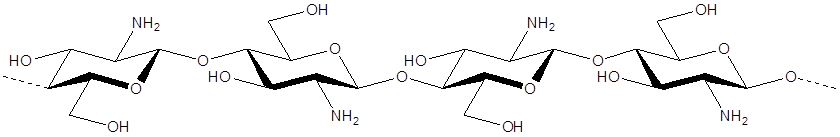
Chitosan: Poly-β-1,4-D-glucosamin
The effectiveness of chitosan is an adequate problem to work on in chemistry classes. Chitosan can easily be gained from chitin, the structural substance of crab- or shrimp shells. From the structural formula it is easy to learn that chitosan is very similar to cellulose: there is only one OH-group per glucose–unit replaced by an amino(NH2)-group. Following cellulose chitin is the second frequently met naturally produced polymeric worldwide, so it's no exotic substance but quite common and with many application opportunities. For teaching chemistry it is also important that it can easily be used to demonstrate structure-property interdependencies.
Bader and Birkholz in their contribution to the Chitin Handbook (R.A.A. Muzzarelli and M.G. Peter, eds. (1997): Chitin Handbook. European Chitin Society) wrote: "The use of polysaccharides as renewable materials is a new subject in chemistry courses. Like in other cases the aim is to show the origin of a product, that has a link with everyday life of the pupils, by developing practical and suitable school experiments for this field (Sommerfeld and Bader 1995/ Insektenpanzer als Rohstoffquelle. Zur Didaktik der Physik und Chemie. Behrendt, H. (ed) Leuchtturm, Alsbach, pp 341. Full version: (1995) c + b (Chemie und Biologie) 39: 18.). In this context the subject chitin is a completion and enlargement. Contrary to former examples, now a polysaccharide is isolated from animal sources for school experiments as well as for industrial use. In addition chitin can be an example for the intelligent use of a waste product, without any conflict of interests, e.g. using it as food or as raw material. Finally, the chitosan made from chitin is a polysaccharide with canonic character (with the possibility to compare it with alginic acid and with neutral polysaccharides like starch or galactomanans.)."
Through a simple process of deacetylation, chitosan (Poly-β-1,4-D-glucosamin) is produced from chitin (Poly-β-1,4-N-acetyl-D-glucosamin):
Deacetylation of chitin:
Summary: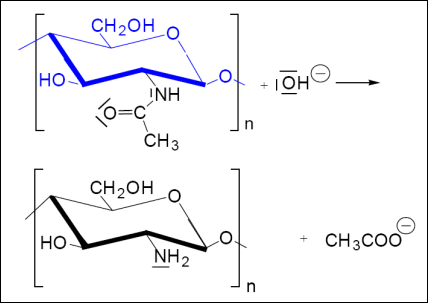
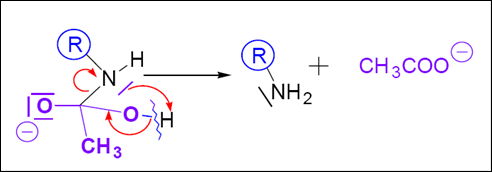
Mechanism: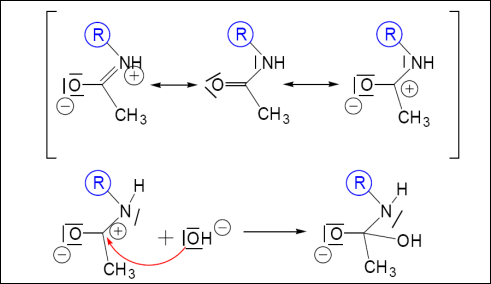

Preparation:
The shells have to be washed with water, dried and grinded.
In a second step the protein needs to be removed with sodium hydroxide solution, and the calcium carbonate with hydrochloric acid.
The obtained chitin will be deacetylised with sodium hydroxide solution, washed with water and dried. The product is a dim pink-beige chitosan looking very similar to chitin.
| Step 1: Shells |
|
|
|
|
Coarsely cleaning and breaking, drying, grinding |
|
|
Step 2: Protein |
|
2 mol/l sodium hydroxide |
|
|
Washing (H2O) |
|
|
Step 2: Calcium carbonate |
|
4 mol/l hydrochloride acid |
|
|
Washing (H2O) |
|
|
Step 3: Deacetylation |
|
50 % sodium hydroxide |
|
|
Washing (H2O) |
|
|
Solute/precipitate |
|
2 % acetic acid |
|
|
Washing (H2O) |
|