Engaging questions:
- What gases are in the fluorescent lamp?
- Why are there no fluorescent lamps filled with the atomic hydrogen?
- What is the working mechanism of the light bulb and the fluorescent lamp?
- Why does the fluorescent lamp not heat up during working?
Equipment:
- spectroscope from the Activity II-1,
- fluorescent lamp.
Description of the Activity :
Students record the white light spectrum with the use of lamp with a typical bulb, then they exchange it for the fluorescent lamp and compare both images.

Figure II.6. A sample spectrum of the fluorescent lamp.
Discussion:
- Why in the spectrum of fluorescent lamp can only some lines be observed, not the entire set of colours?
- The location of particular bands: to what is it related?
- In the Figure II.7 the emission spectrum of hydrogen atoms is presented. Compare this spectrum with the recorded one. What is the reason for similarities and differences?
- How can the hydrogen spectrum be related to the structure of its atom?
- With what process is the light emission by hydrogen connected?
- The spectrum you recorded is the emission spectrum. Think, how the absorption spectrum of hydrogen and of the fluorescent lamp should look like.
- Emission spectrum of hydrogen presented in Figure II.7 is the atomic spectrum. If we fill the fluorescent lamp with hydrogen, it would be in the molecular form (H2). Will the emission spectrum of the hydrogen fluorescent lamp be the same as the presented atomic one?
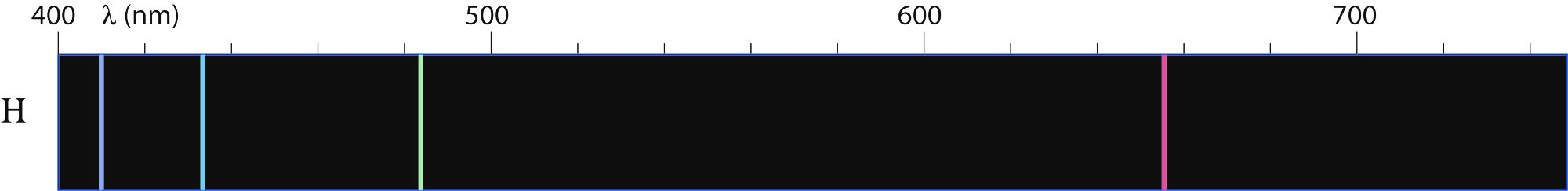
Figure II.7. Emission spectrum of hydrogen atoms. Derived from Physics for Scientists and Engineers (6th ed.) by Serway and Jewett (Thomson Brooks/Cole, 2004).