Engaging questions:
- What will be the colours in the room illuminated by daylight if we shut the window pane with red foil?
- How does the spectrum of the light bulb change after passing through the red filter?
- What should the spectrum of the red light bulbs look like?
Reagents:
- green solution: FeCl2 1 mol/dm3
- red solution: HCl + methyl orange,
- violet solution: KMnO4 (or blue solution: CuSO4 1 mol/dm3)
Equipment: Spectroscope from the Activity II.1, lamp with a bulb, cuvettes.
Description of the Activity :
Students fill the cuvette with the coloured solution. Cuvettes are placed in the spectroscope on the light path, in front of the shutter (Figure II.2). Students collect the spectra.
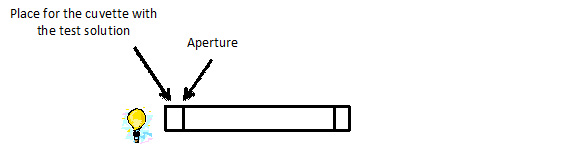
Figure II.3. A diagram of the spectrometer structure together with a place for the sample.
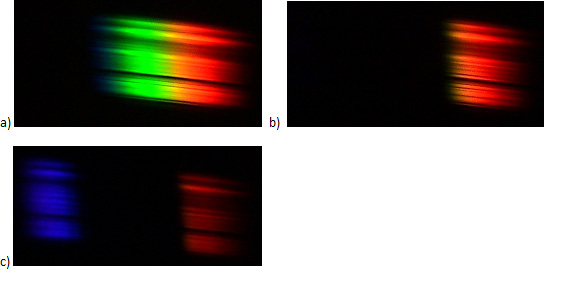
Figure II.4. The spectrum of white light after passing through the solutions: a) FeCl2, b) red food dye, c) KMnO4.
Discussion:
- Compare the colours from the spectra with the solution colours,
- What colours are „missing” in the observed spectra?
- Should the intensity of the solution colours influence the spectra appearance?
- Should the intensity of the bulb light used in the Activity influence the spectra appearance?